Patient case: Tyrvaya® (varenicline solution) as a potential therapy for neuropathic ocular pain associated with trigeminal neuralgia
The contents of this article are informational only and are not intended to be a substitute for professional medical advice, diagnosis, or treatment recommendations. This editorial presents the views and experiences of the author and does not reflect the opinions or recommendations of the publisher of Optometry 360.
By Kaleb Abbott, OD, MS
Introduction
Since the incorporation of neurosensory abnormalities into the Tear Film & Ocular Surface Society’s definition of dry eye disease in 2017, neuropathic ocular pain is increasingly recognized in a subset of dry eye patients and as a contributing factor in specific cases of ocular pain. One source of neuropathic ocular pain is trigeminal neuralgia.1,2 Trigeminal neuralgia is a neuropathic pain condition of the orofacial region defined by the International Classification of Headache Disorders from the International Headache Society by the criteria of paroxysmal attacks of pain affecting the trigeminal nerve; pain characterized by as sudden, superficial, or stabbing; the episodes being similar amongst other patients; and no clinically evident neurological disorder.3 The location of the pain depends on the affected trigeminal branches, as the trigeminal nerve is the largest of the cranial nerves with 3 distinct branches innervating the anterior two-thirds of the face and the top of the head.4
Although trigeminal neuralgia may affect any of the 3 trigeminal nerve branches, it most commonly affects the second and third branches, which innervate the maxillary and mandibular facial areas, respectively.3 However, in cases of first branch involvement, known as the ophthalmic branch, both ocular and periorbital pain may occur in addition to pain at the top of the head.1,2 Ocular pain associated with trigeminal neuralgia may be spontaneous or provoked through gentle palpation of periorbital tissue or eyelids.2,5
Although the true prevalence of trigeminal neuralgia, confounded by convenience sampling, remains unclear, research demonstrates that it primarily affects women older than 60 years.6,7 This article presents a case of neuropathic ocular pain secondary to chronic trigeminal neuralgia with marked improvement in symptoms after initiation of intranasal Tyrvaya® (varenicline solution) as a neurostimulator.
Case report
A 62-year-old woman presented with a long-standing history of trigeminal neuralgia of the right side of her face, as diagnosed 14 years prior. Her pain was in the right eye, periorbitally around the right eye, and on the top of the right side of her head. She exhibited signs of hyperalgesia and allodynia as her symptoms worsened with cold temperatures, exposure to wind, and light touch to the face. The ocular and periocular pain was characterized as shooting or stabbing pain occurring intermittently. She also reported severe photophobia of the right eye only, which was so severe that she reported having to turn down the brightness on computer monitors due to eye pain and having to unscrew lightbulbs at restaurant tables. Her photophobia was consistent with photoallodynia.
Prior trigeminal neuralgia-related procedures included Gamma Knife treatment 14 years prior and microvascular decompression 3 years prior, neither of which improved her symptoms. She also had no improvement with various dosages of pregabalin and gabapentin, and these medications had also caused grogginess. She was currently taking duloxetine 30 mg by mouth once daily with minimal relief. A neuro-ophthalmologist noted V1 through V3 numbness with decreased corneal sensation.
At the initial visit, she had rare corneal staining equally on both eyes; Schimer’s I score of 11 mm and 18 mm for the right and left eye, respectively; a tear break-up time of 5 seconds in each eye; no meibomian gland atrophy via LipiView® meibography (Figure 1); two-thirds of her lower meibomian glands yielding a liquid secretion; clear meibum secretions; and no conjunctival staining of either eye. Ocular surface findings were similar between the right and left eyes, despite her symptoms being unilateral in nature. At that visit, her right eye and right sided facial pain were rated as a 7 to 8 out of 10 using the Wong-Baker Faces Pain Rating Scale. The Ocular Pain Assessment Survey, widely utilized for neuropathic ocular pain, demonstrated severe intensity and frequency of her pain negatively affecting quality of life (Figure 2). The greatest level of pain in the prior 24 hours was reported as a 10 out of 10, while the lowest pain was rated a 3 out of 10, with an average pain reporting of 6 out of 10. The greatest level of pain in the prior 2 weeks was a 10 out of 10, while the lowest pain reported was a 3 out of 10, with an average pain reporting of 6 out of 10. Proparacaine testing demonstrated a 95% improvement in both pain and photoallodynia, indicative of either nociceptive pain or peripheral neuropathic pain.
Topical loteprednol 0.2% was prescribed twice daily in the right eye for 30 days to see if this reduced her ocular pain by alleviating inflammation. At 1-month follow-up, there was only slight improvement in symptoms, so autologous serum eyedrops were prescribed 4 to 6 times daily in the right eye, and the patient was instructed to use 1 drop of loteprednol 0.2% twice daily in the right eye for 3 to 5 days only during flares. A topical immunomodulator was not prescribed in conjunction with the autologous serum eye drops out of concern for possible side effects and the patient’s reported history of eye drop sensitivity.
Two months later, the patient still reported no improvement in symptoms. At that visit, there was strong suspicion of a neuropathic component given the history of trigeminal neuralgia, unilateral nature of her symptoms, and minimal improvement of symptoms with autologous serum eye drops, a topical corticosteroid, and prior punctal plugs. Therefore, corneal nerve imaging was ordered. In-vivo confocal microscopy of her right cornea demonstrated findings consistent with neuropathic corneal pain such as microneuromas and decreased sub-basal plexus nerve density, with unremarkable findings of her left corneal nerves (Figures 3 and 4).
The plan was to continue autologous serum eye drops 4 to 6 times per day in the right eye, loteprednol 0.2% twice daily in the right eye only during flares, and FL-41 tinted glasses during periods of photophobia. This plan was continued because a combination remodeling of the sub-basal nerve plexus with autologous serum eye drops generally takes longer than 2 months, so it was possible that pain would improve with continued therapy. Additionally, there was no intraocular pressure spike noted at this visit.
Three months later, there was still no reduction of right eye pain or periorbital pain on the right side. Intranasal varenicline was prescribed off-label as a neurostimulator to reduce neuropathic pain through trigeminal nerve stimulation. The instructions were to use 1 spray twice daily in each nostril until the next appointment.
At follow-up 3 months later, the patient reported a 60% to 70% improvement in eye pain, eye pressure, periorbital pain, and facial pain. Her pain rating improved to 3 to 4 out of 10 using the Wong-Baker Faces Pain Rating Scale, and the Ocular Pain Assessment Survey questionnaire revealed markedly improved eye pain intensity and frequency with an improvement in quality-of-life score (Figure 5). The worst pain experienced in the prior 24 hours was 8 out of 10, while the least pain was 1 out of 10, with an average pain reporting of 3 out of 10. The worst pain experienced in the prior 2 weeks was 8 out of 10, while the least pain was 1 out of 10, with an average pain reporting of 5 out of 10. There was no change in tear break-up time, meibomian glands yielding a liquid secretion, meibum quality, or corneal staining in either eye at this visit. Approximately 1 month after starting intranasal varenicline, the patient self-discontinued the autologous serum eye drops due to financial burden and loteprednol 0.2% due to no additional pain flares. Therefore, her only prescription ocular treatment during this time was intranasal varenicline. Preservative-free artificial tears were rarely used in the right eye, defined as a couple times per month. Six months later, the patient reported stable ocular and periocular pain with no use of loteprednol 0.2%. Preservative-free artificial tears continued to be used seldomly in the right eye.
Importantly, throughout this follow-up period, there were no changes made to her systemic medication regimen, injections of Botox or nerve blocks, or surgeries performed that could influence her ocular, periocular, or facial pain.
Discussion
Dry eye is a heterogeneous, multifaceted, and complex disorder with various underlying causes that encompass a spectrum of clinical manifestations leading to ocular discomfort. Symptoms may include dryness, burning, foreign body sensation, grittiness, pain, fluctuating vision, or other ocular dysesthesias. However, numerous studies have shown a lack of correlation between ocular signs and patient-reported symptoms.8-13 Additionally, diagnostic testing of tear film parameters often fails to align with patient-reported discomfort.14,15 In certain patients, an explanation for this disconnect may include alterations of trigeminal nerve function, such as neuropathic ocular pain, which can mimic dry eye-related ocular dysesthesias or pain despite nociceptors not being responsible for such symptoms.16
Pain is broadly defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” Chronic pain, outlined by the National Health Interview Survey as daily pain experienced over the past 3 months, is estimated to affect 11.2% of adults in the United States, amounting to over 25 million individuals.17 Pain is often categorized into 2 groups: nociceptive or neuropathic.
In the context of ocular surface disease, nociceptive pain, or dry eye, arises from actual or threatened damage to the ocular surface, particularly the cornea. Conversely, neuropathic pain stems from a disease or lesion of the somatosensory nervous system. Systemic neuropathic pain entities with an underlying etiology include diabetic neuropathy, postoperative pain, or post-herpetic trigeminal neuralgia.18 Meanwhile, there are other recognized chronic pain conditions with ill-defined pathophysiology such as fibromyalgia, irritable bowel syndrome, and atypical facial pain.19 While neuropathic pain may be diagnosed with a demonstratable lesion—like the corneal confocal microscopy performed on this patient, which showed right corneal nerve abnormalities—often a neuropathic pain diagnosis is made clinically.
Neuropathic pain-related symptoms, such as burning, shooting, pins and needles sensations, or itchiness, often manifest spontaneously and can be continuous or episodic.20,21 These patients tend to have lower pain thresholds and higher pain intensities compared with those with nociceptive pain.22,23 The presence of neuropathic ocular pain can be differentiated from traditional dry eye in cases of specific clinical features, including:
- Lack of response to traditional dry eye therapies19,24
- Disproportionate dry eye symptoms compared with ocular surface signs1,18,21,25
- Coexistence of non-ocular chronic pain conditions26
- Presence of spontaneous burning, hyperalgesia (eg, sensitivity to wind), and allodynia (eg, sensitivity to light)21 indicative of altered somatosensory function27,28
- Pain involving the periorbital region or eyelids (secondary cutaneous allodynia)21
Dry eye frequently coexists with other chronic pain syndromes.24,26 The presence of a diagnosed systemic chronic pain condition is associated with more severe dry eye symptoms and is the most significant predictor for dry eye symptoms greatly outweighing ocular surface signs.8,29 Likewise, dry eye symptoms correlate more strongly with non-ocular pain than tear film parameters, resembling non-ocular peripheral neuropathic pain where symptoms occur without identifiable tissue irregularities.25,30
All of this suggests that dry eye is associated not only with local somatosensory dysfunction of the corneal nerves but systemic somatosensory dysfunction as well. For this reason, it is plausible that some dry eye may be a peripheral manifestation of systemic disease and respond better to systemic treatments targeting underlying somatosensory dysfunction as opposed to topical treatments for nociceptive pain.
While this patient demonstrated some evidence of ocular surface dryness, her symptoms were clearly disproportionate to her ocular surface signs and unilateral in nature despite having relatively equal ocular surface signs between the eyes. She exhibited evidence of allodynia in the form of photoallodynia where normal indoor lights would elicit debilitating pain. She also reported extreme ocular discomfort in cold and windy environments, which is consistent with hyperalgesia. Her neurologist had also noted tactile allodynia where pain could be evoked with light touch to the skin. This indicated a potential neuropathic mechanism as an explanation for her ocular symptoms, which seemed plausible given the history of trigeminal neuralgia on the side in which she was experiencing her ocular pain. Like non-ocular neuropathic pain (eg, diabetic neuropathy), patients like this one may demonstrate reduced corneal sensation (hypoesthesia) with simultaneous hyperalgesia and heightened evoked pain, consistent with her reduced V1-V3 sensation diagnosed by a neurologist.16,31-33
It is not out of the question that trigeminal nerve stimulators could be useful in the alleviation of neuropathic ocular pain as trigeminal nerve stimulators have been shown to reduce pain intensity, light sensitivity, wind sensitivity, and burning sensation in cases of trigeminal neuralgia.34 Transcutaneous electrical nerve stimulation has been used since the 1970s to decrease nerve pain using the principles of gate control theory.34 This theory describes chronic pain states occurring in cases where, despite no nerve stimulation, the body continues to open gates within the peripheral nervous system resulting in amplified pain signaling.35 Neurostimulation aims to neuromodulate pain by producing a signal within a nerve resulting in the blockages of nerve gates in the peripheral nervous system.34,36 This concept is not new to eye care, as intranasal electrical neurostimulation has been reported to be effective in the management of neuropathic corneal pain.37 What differentiates intranasal varenicline, in this case, is that the stimulation is through activation of the trigeminal parasympathetic pathway through a highly selective cholinergic agonist binding to the cholinergic receptors on trigeminal sensory nerve endings within the nose, thereby stimulating the trigeminal nerve.38 Interestingly, nicotinic receptor agonists, like varenicline, have been indicated as potential treatments for neuropathic pain.39 Another mechanism of action for which varenicline may have improved pain in this patient is through mitigation of nociceptive stimulation by augmenting lacrimal gland secretions. However, this does not seem like a plausible explanation for her unilateral improvement in ocular and facial pain given the symmetric ocular surface findings and minimal improvement with autologous serum eye drops, which have similar components to natural human tears.40 While a combination of autologous serum eye drops with a topical immunomodulator has been shown to remodel the corneal sub-basal nerve plexus in as little as 4 to 6 months, this patient only utilized autologous serum eye drops for roughly 3 months and was not using a topical immunomodulator.41 Her supply of serum drops lasted 3 months, and she did not pursue a blood draw and serum drop refill given the financial burden and notable improvement in symptoms with intranasal varenicline dosed twice daily. The stable symptoms at the 9-month mark after initiation of intranasal varenicline suggest the durability of this treatment. Unfortunately, in-vivo confocal microscopy was not performed after the baseline imaging due to the imaging apparatus requiring repair.
Conclusion
Treatment options for trigeminal neuralgia-associated neuropathic ocular pain remain quite limited with symptomatic improvement being difficult to achieve with ocular treatments. This case demonstrates the potential for intranasal varenicline to be used off-label in the treatment of neuropathic ocular pain in patients with trigeminal neuralgia. Future research is needed to better understand the utility of such a treatment in neuropathic ocular pain and the mechanisms involved in the reduction of ocular, periocular, and facial pain.
Takeaway Points
- Trigeminal neuralgia, when affecting the ophthalmic branch (V1), may result in severe ocular pain. This pain can be spontaneous or provoked by stimuli and may not align with conventional dry eye signs, emphasizing the need to recognize neuropathic components in ocular pain syndromes.
- A significant disconnect was observed between the patient’s severe ocular symptoms and the relatively normal ocular surface findings. This highlights that traditional dry eye diagnostic tests may not always reflect the true extent of pain or discomfort in patients with neuropathic ocular pain.
- The case study demonstrates that intranasal varenicline, used off-label as a neurostimulator, may significantly improve symptoms of neuropathic ocular pain in patients with trigeminal neuralgia. After initiating this treatment, the patient experienced a 60% to 70% reduction in ocular pain, highlighting the potential of varenicline for managing neuropathic ocular pain.
Figure 1. Meibography (LipiView®) demonstrates no meibomian gland atrophy or structural abnormalities in the right lower lid and the left lower lid.
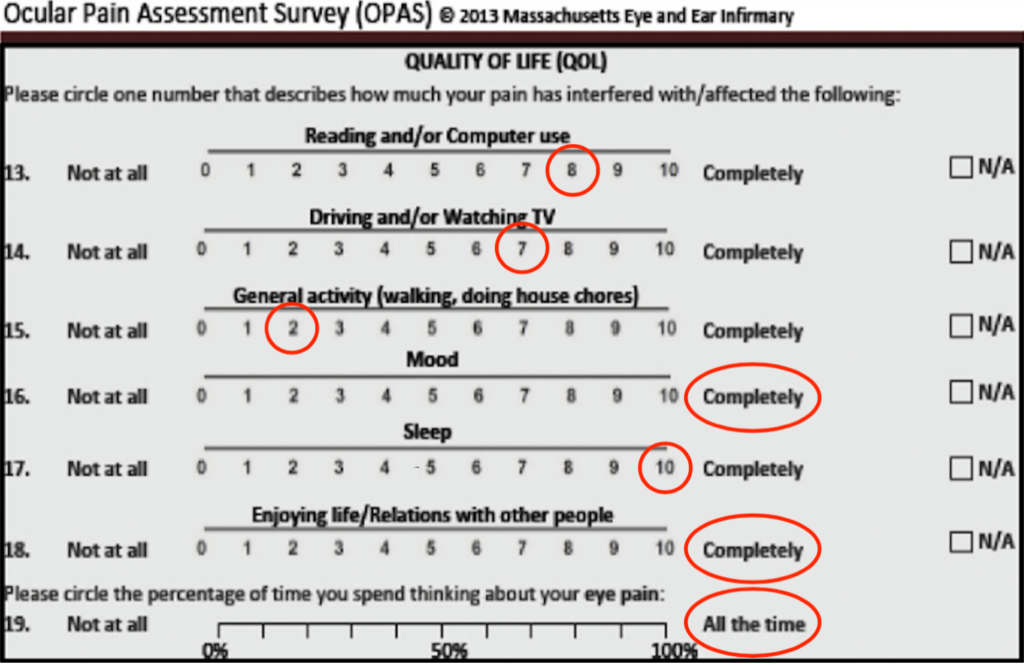
Figure 2. Quality of life section of the Ocular Pain Assessment Survey demonstrates baseline degree in which pain was affecting the patient’s activities of daily living.
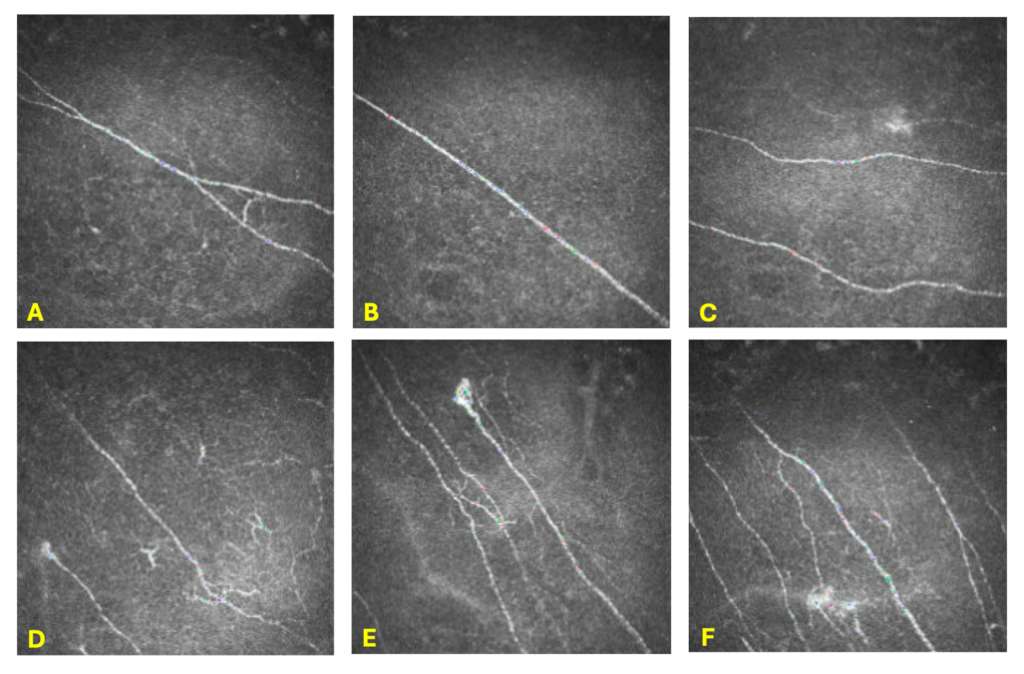
Figure 3. In-vivo confocal microscopy of the corneal sub-basal nerve plexus in the right eye taken with a Heidelberg HRT3. Pictures A-C depict decreased nerve density. Pictures C-F demonstrate microneuromas. Pictures D-F detail nerve tortuosity, nerve beading, and nerve sprouting.
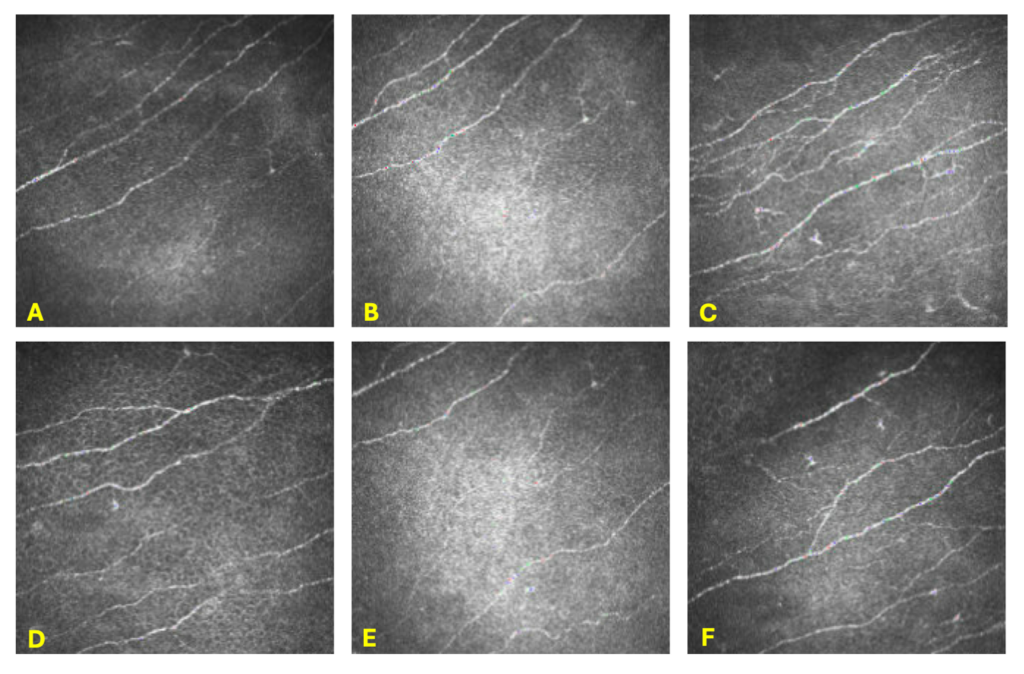
Figure 4. In-vivo confocal microscopy of the corneal sub-basal nerve plexus in the left eye taken with a Heidelberg HRT3. Pictures A-F depict a normal sub-basal nerve plexus. Pictures C and F demonstrate mild Langerhans cells.
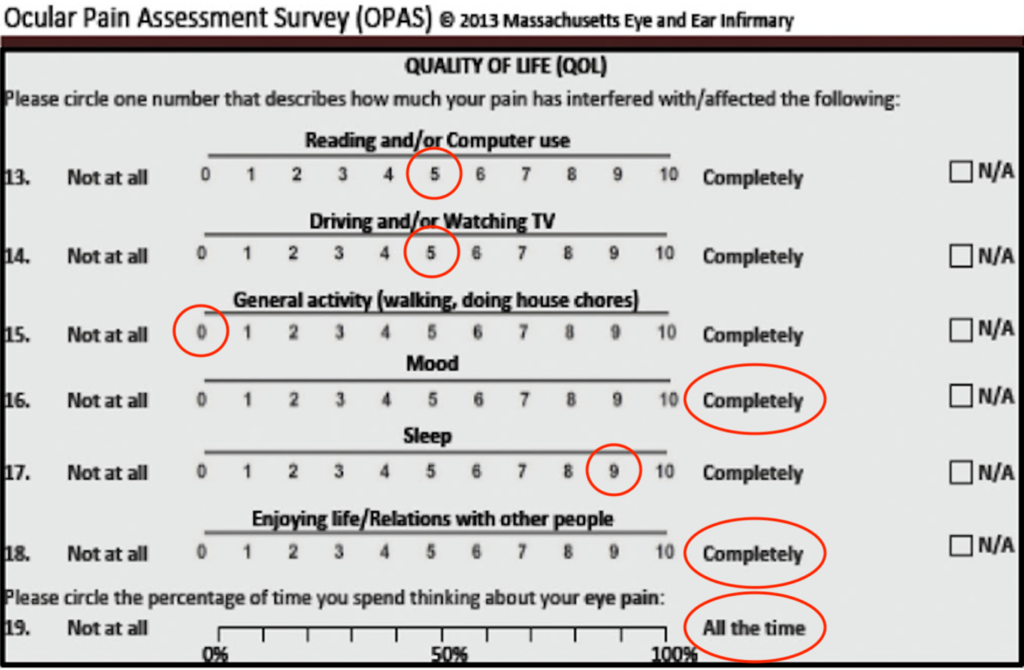
Figure 5. Quality of life section of the Ocular Pain Assessment Survey demonstrates improvements in pain interfering with the patient’s activities of daily living.
Kaleb Abbott, OD, MS, is from the Department of Ophthalmology at the University of Colorado School of Medicine. He can be reached at Kaleb.abbott@cuanschutz.edu.
References
- Brazis PW, Lee AG, Stewart M, Capobianco D. Clinical review: the differential diagnosis of pain in the quiet eye. Neurologist. 2002;8(2):82-100. doi:10.1097/00127893-200203000-00003
- Mehra D, Cohen NK, Galor A. Ocular surface pain: a narrative review. Ophthalmol Ther. 2020;9(3):1-21. doi:10.1007/s40123-020-00263-9
- Headache Classification Committee of the iNternational Headache Society. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. doi:10.1177/0333102413485658
- Araya EI, Claudino RF, Piovesan EJ, Chichorro JG. Trigeminal neuralgia: basic and clinical aspects. Curr Neuropharmacol. 2020;18(2):109-119. doi:10.2174/1570159X17666191010094350
- Di Stefano G, Maarbjerg S, Nurmikko T, Truini A, Cruccu G. Triggering trigeminal neuralgia. Cephalalgia. 2018;38(6):1049-1056. doi:10.1177/0333102417721677
- Siqueira SR, Teixeira MJ, Siqueira JT. Clinical characteristics of patients with trigeminal neuralgia referred to neurosurgery. Eur J Dent. 2009;3(3):207-212.
- De Toledo IP, Conti Réus J, Fernandes M, et al. Prevalence of trigeminal neuralgia: a systematic review. J Am Dent Assoc. 2016;147(7):570-576.e2. doi:1016/j.adaj.2016.02.014
- Vehof J, Smitt-Kamminga NS, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology. 2017;124(3):280-286. doi:10.1016/j.ophtha.2016.11.008
- Galor A, Feuer W, Lee DJ, et al. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013;54(2):1426-1433. doi:10.1167/iovs.12-10819
- Martinez JD, Galor A, Ramos-Betancourt N, et al. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin Ophthalmol. 2016;10:1335-1342. doi:10.2147/opth.S106451
- Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol. 2014;92(2):161-166. doi:10.1111/aos.12012
- Kyei S, Dzasimatu SK, Asiedu K, Ayerakwah PA. Association between dry eye symptoms and signs. J Curr Ophthalmol. 2018;30(4):321-325. doi:1016/j.joco.2018.05.002
- Schmidl D, Witkowska KJ, Kaya S, et al. The association between subjective and objective parameters for the assessment of dry-eye syndrome. Invest Ophthalmol Vis Sci. 2015;56(3):1467-1472. doi:10.1167/iovs.14-15814
- Lanza NL, McClellan AL, Batawi H, et al. Dry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul Surf. 2016;14(2):216-223. doi:10.1016/j.jtos.2015.12.007
- Yeh TN, Graham AD, Lin MC. Relationships among tear film stability, osmolarity, and dryness symptoms. Optom Vis Sci. 2015;92(9):e264-e272. doi:10.1097/opx.0000000000000649
- Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol. 2015;99(5):665-668. doi:10.1136/bjophthalmol-2014-306057
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769-780. doi:10.1016/j.jpain.2015.05.002
- Smith BH, Hébert HL, Veluchamy A. Neuropathic pain in the community: prevalence, impact, and risk factors. Pain. 2020;161 Suppl 1:S127-S137. doi:10.1097/j.pain.0000000000001824
- Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev. 2015;11(2):70-85. doi:10.2174/157339711102150702112236
- Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248-257. doi:10.1016/j.pain.2003.12.024
- Galor A, Moein HR, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31-44. doi:10.1016/j.jtos.2017.10.001
- Staud R, Weyl EE, Riley JL 3rd, Fillingim RB. Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS One. 2014;9(2):e89086. doi:10.1371/journal.pone.0089086
- Maier C, Baron R, Tölle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439-450. doi:10.1016/j.pain.2010.05.002
- Galor A, Covington D, Levitt AE, et al. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain. 2016;17(3):310-318. doi:10.1016/j.jpain.2015.10.019
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959-1964. doi:10.1016/s0140-6736(99)01307-0
- Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98(12):1712-1717. doi:10.1136/bjophthalmol-2014-305201
- Spierer O, Felix ER, McClellan AL, et al. Corneal mechanical thresholds negatively associate with dry eye and ocular pain symptoms. Invest Ophthalmol Vis Sci. 2016;57(2):617-625. doi:10.1167/iovs.15-18133
- Galor A, Levitt RC, McManus KT, et al. Assessment of somatosensory function in patients with idiopathic dry eye symptoms. JAMA Ophthalmol. 2016;134(11):1290-1298. doi:10.1001/jamaophthalmol.2016.3642
- Vehof J, Smitt-Kamminga NS, Kozareva D, Nibourg SA, Hammond CJ. Clinical characteristics of dry eye patients with chronic pain syndromes. Am J Ophthalmol. 2016;162:59-65.e2. doi:10.1016/j.ajo.2015.11.017
- Galor A, Felix ER, Feuer W, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015;99(8):1126-1129. doi:10.1136/bjophthalmol-2014-306481
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599-1606. doi:10.1097/j.pain.0000000000000492
- Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48(1):173-181. doi:10.1167/iovs.06-0127
- Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46(7):2341-2345. doi:10.1167/iovs.04-1426
- Mehra D, Mangwani-Mordani S, Acuna K, et al. Long-term trigeminal nerve stimulation as a treatment for ocular pain. Neuromodulation. 2021;24(6):1107-1114. doi:1111/ner.13402
- Abd-Elsayed AA, Grandhi R, Sachdeva H. Effective management of trigeminal neuralgia by neurostimulation. Ochsner J. 2015;15(2):193-195.
- Rosenberg M, Curtis L, Bourke DL. Transcutaneous electrical nerve stimulation for the relief of postoperative pain. Pain. 1978;5(2):129-133. doi:10.1016/0304-3959(78)90034-9
- Dieckmann G, Koseoglu ND, Akhlaq A, Pondelis N, Hamrah P. Efficacy of intranasal neurostimulation for peripheral pain among neuropathic corneal pain patients. Presented at the 2018 ARVO Annual Meeting, April 29-May 3, 2018, Honolulu, Hawaii.
- Frampton JE. Varenicline solution nasal spray: a review in dry eye disease. Drugs. 2022;82(14):1481-1488. doi:10.1007/s40265-022-01782-4
- Hone AJ, McIntosh JM. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018;592(7):1045-1062. doi:10.1002/1873-3468.12884
- Vazirani J, Sridhar U, Gokhale N, et al. Autologous serum eye drops in dry eye disease: preferred practice pattern guidelines. Indian J Ophthalmol. 2023;71(4):1357-1363. doi:10.4103/ijo.Ijo_2756_22
- Aggarwal S, Colon C, Kheirkhah A, Hamrah P. Efficacy of autologous serum tears for treatment of neuropathic corneal pain. Ocul Surf. 2019;17(3):532-539. doi:10.1016/j.jtos.2019.01.009

Contact Info
Grandin Library Building
Six Leigh Street
Clinton, New Jersey 08809


